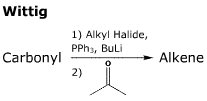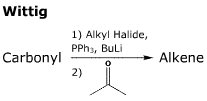
- In formation of the ylid, a primary or secondary bromine or iodine substituted halide is used.
- This first step is an SN2 reaction.
- A strong base, BuLi or NaH is used to form the ylid.
- Formation of the four-membered ring effectively progresses via. a [2+2] cycloaddition.
- The second stage of the reaction is driven by formation of the very strong P-O bond(s).
- The P=O bond is extremely strong, and its formation is often a driving force in reactions where it is possible. For other examples see the Bischler-Napieralski reaction, the Mitsunobu reaction and the Vilsmeier reaction.
- If the negative charge on the ylid can be delocalised, it is known as a stabilised ylid. These preferentially form the E-alkene. Unstabilised ylids form the Z-alkene.
References:
- Balema V.P.; Wiench J.W. et al; J. A. Chem. Soc., 2002, Volume 124, Issue 22, 6244-6245
- Greenwood E.S.; Hitchcock P.B.; Parsons P.J.; Tetrahedron, 2003, Volume 59, Issue 18, 3307-3314