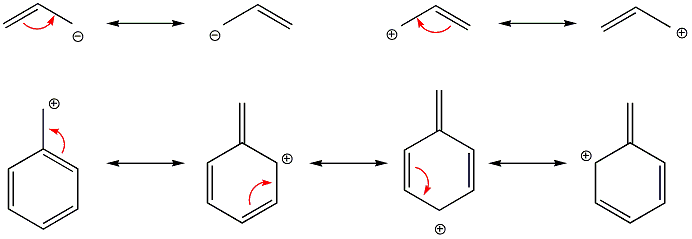
In systems with double bonds, or those with charges, the electron density (and hence charge) is delocalised over several atoms in the molecule. This adds to the stability of these compounds, as each atom itself experiences less than, for example, a whole positive charge. The different structures that could be drawn show the same compound, and are only convenient representations for arrow drawing purposes. The delocalisation arrow is used in these cases to show that the compound is not changing in any way.