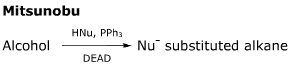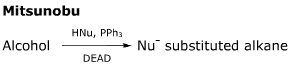
- The anion formed in the first step is stabilised by electron delocalisation into the adjacent ester group.
- The P=O bond is extremely strong, and its formation is often a driving force in reactions where it is possible. For other examples see the Bischler-Napieralski reaction, the Vilsmeier reaction and the Wittig reaction.
- The final step is a simple SN2 reaction, which takes advantage of the strength of the P-O bond formed earlier in the reaction.
- The configuration of the starting alcohol is inverted by the course of this reaction.
References:
- Dandapani S.; Curran D.P.; Tetrahedron, 200, Volume 58, Issue 20, 3855-3864
- Gao Y.; Lane-Bell P.; Vederas J.C.; J. Org. Chem., 1998, Volume 63, Issue 7, 2133-2143