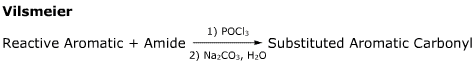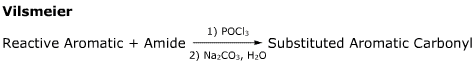
- This reaction gives good yields of monosubstituted products, in absence of strong acid or Lewis acid.
- The P=O bond is extremely strong, and its formation is often a driving force in reactions where it is possible. For other examples see the Bischler-Napieralski reaction, the Mitsunobu reaction and the Wittig reaction.
- This reaction is a substitute for the Friedel-Crafts acylation.
- The iminium cation reacts with pyrrole to form a more stable iminium salt. The extra stabiliy comes from the conjugation between the pyrrole nitrogen and the iminium group.
References:
- Soledad M.; Pedras C.; Zaharia I.L.;
Organic Letters, 2001, Volume 3, Issue 8, 1213-1216
- Brenna E.; Fuganti C.; Dei Negri C.; Gatti F.G.; Serra S.; Tetrahedron Assymmetry, 2004, Volume 15, Issue 2, 335-340