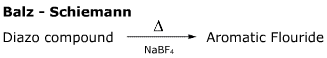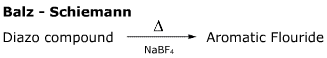
- The first step of this reaction, loss of nitrogen, is via. the SN1 mechanism.
- Diazo compounds are often prone to loss of nitrogen, because the triple bond is so strong. Formation of this bond is the driving force for many nitrogen functional group reactions, for example the Sandmeyer reaction, the Semi-Pinacol reaction, the Tiffeneau-Demjanov reaction and the Wolff-Kischner reaction.
- Boron acts as a fluoride donor in this reaction.
References:
-
Kiryanov A.; Seed A.; Sampson P.; Tetrahedron Letters, 2001, Volume 42, Issue 50, 8797
- Laali K.; Geltwert V.; Journal of Fluorine Chemistry, 2001, Volume 107, Issue 1, 31-34