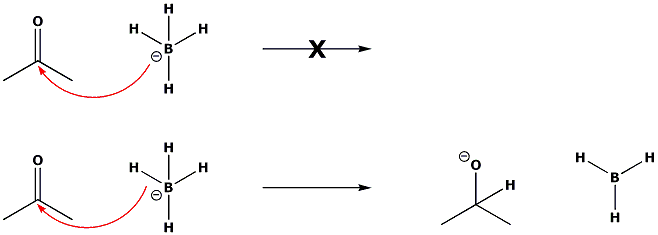
Boron in its 3-coordinate form has a vacant p-orbital. It can accept a pair of electrons from a Lewis base to give overall neutral species or form a tertahedral anion with a nucleophile. These species can no longer accept electrons as Boron has no more vacant orbitals to accomodate them. Hence 4-coordinate boron never forms 5-coordinate boron, instead donating the electrons from one of its bonds and delivering one of its substituents to an electrophilic centre. The group can be H, in which case the reaction is hydride donation.