
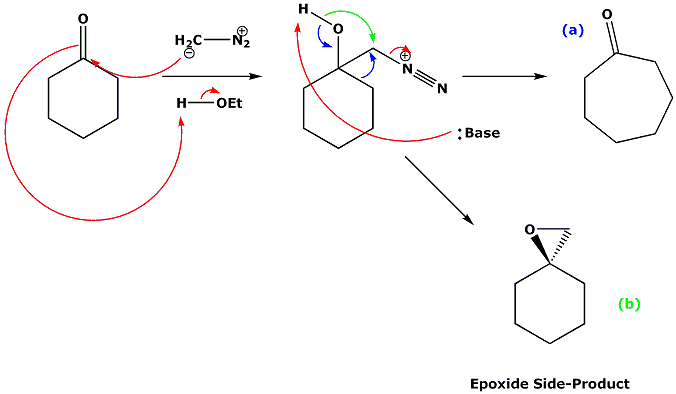
- There are two versions of this reaction, both forming a ring-expanded ketone. The second version uses a diazo group to eject nitrogen from the compound.
- Diazo compounds are often prone to loss of nitrogen, because the triple bond is so strong. Formation of this bond is the driving force for many nitrogen functional group reactions, for example the Balz-Schiemann reaction, the Sandmeyer reaction, the Semi-Pinacol reaction and the Wolff-Kischner reaction.
- The second type of Tiffeneau-Demjanov reactions are Semi-Pinacol systems using diazonium salts which have been derived from 2-amino alcohols.
- This second version of the reaction can also form an epoxide side-product. In small rings this epoxide can itself form a ring-expanded ketone.
References:
- McClure M.; Schiesser C.; White J.; Australian Journal of Chemistry, 2004, Volume 57, Issue 9,
869-876
- Houdai T.; Matsuoka S.; Murata M.; Satake M.; Ota S.; Oshima Y.; Rhodes L.L.; Tetrahedron, 2001, Volume 57, Issue 26, 5551-5555


