|
From the table in the appendix , you should be able to verify the following for this reaction: DH0 = -673 kcal DS0 = +43.3 kcal deg-1 (-TD S0 = -13 kcal) DG0 = -686 kcal A large quantity of heat, 673 kcal, is given off during the burning of glucose because of the large number of bonds formed between oxygen and other atoms during the reaction. At the same time, one mole of a solid is converted to six moles of a liquid, so the entropy, or disorder effect, is appreciable. (The 02 and CO2 gases have similar entropies.) Entropy gives the glucose reaction an extra 13-kcal push. |
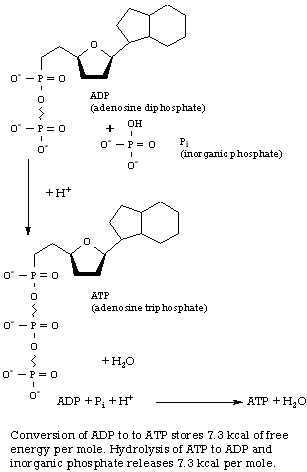
|