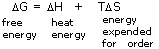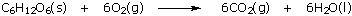|
As has been stated, the key to spontaneity in chemical reactions is a decrease in free energy, G, and not simply in the total energy or enthalpy, H. This residual free energy is the total thermal energy minus any energy that is required to create order:
In the combustion of ethanol, 327 kcal of energy are given off per mole as heat, but not all 327 kcal can be used for outside work. The equivalent of 10 kcal of heat is the price that must be paid in entropy when one mole of liquid and three of gas are transformed by reaction into three moles of liquid and only two of gas. It is the loss of one mole of gas that is critical from an entropy standpoint. If all of the chemical energy of the ethanol combustion is dissipated as heat, 327 kcal will be given off. If we couple this combustion to another, nonspontaneous reaction and use the driving force of the ethanol reaction to make the second process take place, we can use only 317 of the 327 kcal as this driving force. This is what the "free" in free energy means. |
Living organisms depend on coupled reactions. They use energyproducing reactions to synthesize compounds that are high in free energy and to make a great many energetically unfavorable reactions run "uphill." Enzymes are the keys to this coupling. They bind reactant and product molecules selectively to their surface, and ensure that when a "downhill" reaction occurs, the "uphill" reaction accompanies it so that a minimum of energy is wasted as emitted heat. The main energy-producing reaction in all oxygen-using living organisms is combustion of the sugar glucose:
|