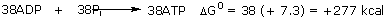|
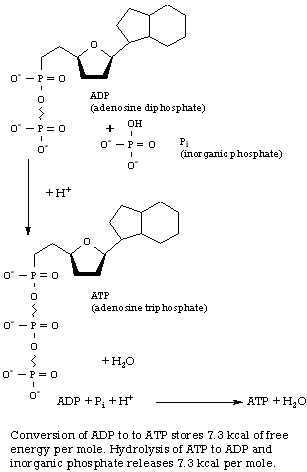
In a living cell, glucose combustion is connected to the machinery for synthesizing a special energy-storage molecule, adenosine triphosphate, or ATP (right), which we saw in Chapter 10. This coupling occurs via an elaborate set of reactions-fermentation, citric acid cycle, and respiratory pathway-using more than twenty different enzymes in succession. The sole purpose of this complicated metabolic system is to ensure that as much of the 686 kcal of free energy as possible is stored as ATP molecules rather than being dissipated as heat. Each ATP molecule synthesized from ADP and inorganic phosphate (represented by Pi) stores 7.3 kcal mole-l of free energy for later use: The important feature about ATP is its universality. All energy-producing reactions in living organisms store their energy in ATP, and all energy-requiring reactions obtain this energy from ATP. The ultimate uses and the origins of any particular packet of energy in the organism are thereby separated. Not all of the free energy liberated by the glucose reaction can be saved. The best that has been achieved in three billion years of evolution of life on this planet is the synthesis of 38 molecules of ATP for every molecule of glucose burned:
|
|