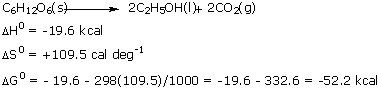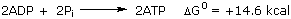|
The overall reaction still liberates enough free energy to be highly spontaneous:
It may look bad that only 277/686 or 40% of the available free energy of the glucose reaction is saved. However, the 409 kcal of energy that are dissipated are not useless. They make the reaction spontaneous and ensure that it never can proceed the wrong way. The situation is analogous to that of a water-mill operator who is content to take a smaller amount of energy out of a rushing stream to power his mill in return for the guarantee that the stream will never back up. If yeast is given a plentiful supply of oxygen, it will burn glucose and produce energy by the overall reaction just given. But if oxygen is cut off, yeast can do what we cannot. Yeast can shut down its citric acid cycle and respiratory chain, and continue to produce energy on a smaller scale by fermenting glucose to ethanol: |
More free energy drive is provided by the disordering of products (32.6 kcal) than by the liberation of heat (19.6 kcal)! Much less energy is saved in this fermentation; only two molecules of ATP are synthesized instead of 38:
|