|
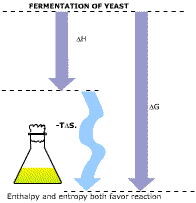
The ATP synthesis comes close to using more chemical energy than was provided by the heat of reaction. This does not matter; entropy is just as effective as enthalpy in driving other reactions. What does matter is the overall DG. The vector energy diagram for glucose fermentation in yeast is shown at the upper right. Enthalpy and entropy arrows both point downward, but the entropy arrow is longer. We now can go back to the example of gravitational energy-the rock rolling down a hillside (middle right). This is a special case in which the entropy change is negligible. A hill with a rock at the top has the same degree of disorder, or entropy, as a hill with a rock at the bottom. The relationship D G = D H - TD S still holds, but D S is zero. In such a situation it is correct to say that spontaneous processes lead to lower energy, but it must be kept in mind that this is only a special case of the general relationship. |
|