|
11. The work function (see Problem 10) for lithium metal
is 55.7 kcal 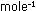 .
How does this compare with the first ionization energy of gaseous
Li atoms? What is the longest-wavelength light that could be used
in a lithium photocell? .
How does this compare with the first ionization energy of gaseous
Li atoms? What is the longest-wavelength light that could be used
in a lithium photocell?
12. Copper has a work function of 92.3 kcal 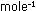 .
Can electrons be ejected from copper by visible light? If not, what
types of radiation could? .
Can electrons be ejected from copper by visible light? If not, what
types of radiation could?
13. The maximum wavelength for emission from a cesium photocell
is 6200 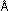 .
What is the work function for cesium metal? .
What is the work function for cesium metal?
14. In the Bohr model of the hydrogen atom, what is the radius
of the electron orbit for quantum number n=2? What is the energy
of this quantum state? What is the energy of state n=3?
15. What is the energy difference between states n=2 and
n=3 of the hydrogen atom? What is the wavelength of radiation absorbed
when the hydrogen atom jumps from n=2 to n=3? In what part of the
electromagnetic spectrum does this occur?
16. Calculate the wavelengths of the hydrogen atom transitions
from =2 to
n=3, 4, 5, 6, 7, and  (infinity). Plot these along a wavelength scale. Notice how the
lines become closer and closer together as the upper quantum number
n increases.
(infinity). Plot these along a wavelength scale. Notice how the
lines become closer and closer together as the upper quantum number
n increases.
|
|