|
6.
The triple bond energies in acetylene, hydrogen cyanide, and nitrogen,
are 194, 213, and 226 kcal mole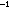 ,
respectively. Diagram these three molecules, showing the positions
of all bonds and lone electron pairs. Why would one expect the observed
progression of bond energies? In singly bonded -N-N-N-N- chains,
it was stated previously that these were unstable in comparison
with -C-C-C-C- chains because of repulsions between nitrogen lone
electron pairs. Why is this apparently not a dominant factor in
the comparison of HC ,
respectively. Diagram these three molecules, showing the positions
of all bonds and lone electron pairs. Why would one expect the observed
progression of bond energies? In singly bonded -N-N-N-N- chains,
it was stated previously that these were unstable in comparison
with -C-C-C-C- chains because of repulsions between nitrogen lone
electron pairs. Why is this apparently not a dominant factor in
the comparison of HC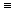 CH,
HCN, and N CH,
HCN, and N ?
Where are the lone pairs positioned in the N ?
Where are the lone pairs positioned in the N molecule?
molecule?
7. The conventional numbering system for a three-ring carbon
skeleton such as that of alizarin is
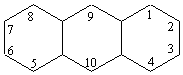
Hence the undissociated alizarin molecule shown previously in this
chapter can be described as having carboryl (C=0) groups at Positions
9 and 10, and -OH groups at Positions 1 and 2. The resonance model
for the alizarin ion shown in this chapter has -0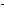 groups at Positions 1 and 2. Draw another resonance structure with
-0
groups at Positions 1 and 2. Draw another resonance structure with
-0 groups at Positions 9 and 10 and carbonyl groups at 1 and 2. What
would this imply about shifts of electrons to and from the various
oxygen atoms?
groups at Positions 9 and 10 and carbonyl groups at 1 and 2. What
would this imply about shifts of electrons to and from the various
oxygen atoms?
|
|