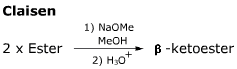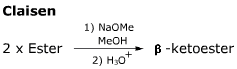
- In the first step of the reaction an enolate is formed by a tautomerism.
- Crossed reactions can only be performed if one component is more electrophilic and cannot enolise.
- This reaction is by its nature intermolecular. The version which involves an intramolecular reaction is called the Dieckmann reaction.
- When the ketoester is first formed, base is still present so a further reaction to form the enolate takes place. Acidic workup leaves the b-ketoester product.
- The Enolate formed in this reaction in the second to final step is stabilised by electron delocalisation, the negative charge being spread across both 'carbonyl' groups.
References:
- Hua D.H.; Chen Y.; Sin H.S.; Maroto M.J.; Robinson P.D.; Newell S.W.; Perchellet E.M.; Ladesich J.B.; Freeman J.A.; Perchellet J.P.; Chiang P.K.; J. Org. Chem., 1997, Voulme 62 , Issue 20, 6888-6896
- Flanagan S.R.; Harrowven D.C.; Bradley M.; Tetrahedron, 2002, Volume 58, Issue 30, 5989-6001