Tautomers are isomers of a compound which differ only in the position of the protons and electrons. The carbon skeleton of the compound is unchanged. A reaction which involves simple proton transfer in an intramolecular fashion is called a tautomerism.
Keto-enol tautomerism is a very common process, and is acid or base catalysed. Typically the 'keto' form of the compound is more stable, but in some instances the 'enol' form can be the more stable.
Some examples of tautomerism:
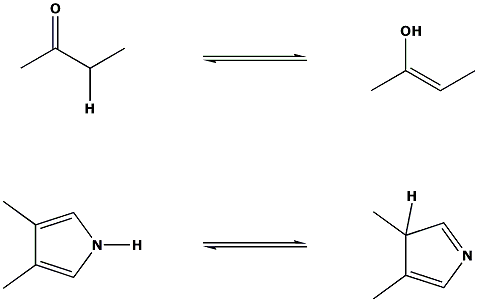
NOTE: The equilibrium arrows above do not intend to show the position of the equilibrium, only that an equilibrium exists between the two forms.