|
Description
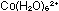 is
pink in aqueous solution and is
pink in aqueous solution and 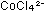 is blue. The equilibrium between these two species can be disturbed
by adding
is blue. The equilibrium between these two species can be disturbed
by adding 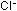 ions and by changing the temperature. The changes in the equilibrium
position are as predicted by Le Chatelier's principle.
ions and by changing the temperature. The changes in the equilibrium
position are as predicted by Le Chatelier's principle.
Apparatus
Six boiling tubes and a rack.
One 100cm measuring cylinder.
measuring cylinder.
Three 250cm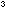 beakers.
beakers.
Two dropping pipettes
Access to a top pan balance.
|
|
Chemicals
The quantities given are for one demonstration.
4g cobalt(II) chloride-6-water - 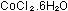
100cm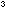 of
concentrated hydrochloric acid. of
concentrated hydrochloric acid.
About 200cm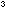 of crushed ice.
of crushed ice.
Method
Before the Demonstration
Boil a beaker of water and prepare a beaker of crushed ice and water.
Dissolve about 4g of cobalt(II) chloride-6-water in 40cm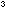 of water. A pink solution containing
of water. A pink solution containing 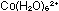 will be formed.
will be formed.
|