|
The Demonstration
Take the pink cobalt chloride solution and make it up to 100cm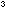 with concentrated hydrochloric acid using a measuring cylinder.
A violet solution will be formed. Adding more hydrochloric acid
will produce a blue solution containing
with concentrated hydrochloric acid using a measuring cylinder.
A violet solution will be formed. Adding more hydrochloric acid
will produce a blue solution containing 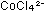 ,
while adding water will restore the pink colour. ,
while adding water will restore the pink colour.
By trial and error produce an 'in between' violet coloured solution
containing the two cobalt chloride ions and place about 2cm depth
of it in each of the six boiling tubes. Place these on the bench
in two groups of three.
|
|
1. Effect of concentration
Keeping one tube as a control, add water to one tube and concentrated
hydrochloric acid to the other using dropping pipettes. If desired,
show that these changes aer reversible.
2. Effect of Temperature
Keeping one tube as a control, place another tube in hot water (over
90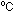 ). It will
go blue. Put the third tube in the ice/water mixture. It will go
pink. If desired, shoe that these chenges are reversible. ). It will
go blue. Put the third tube in the ice/water mixture. It will go
pink. If desired, shoe that these chenges are reversible.
|