|
Ammonia
Synthesis: Incomplete Reaction
The ammonia synthesis reaction is less extreme than 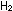 combustion:
combustion:
 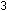 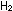 (g) +
(g) + 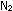 (g)
(g) 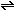 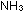 (g)
(g)
 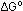 = -7.95 kcal per 2 moles of
= -7.95 kcal per 2 moles of 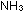
The equilibrium constant is not the enormous exponential value that
we saw for the HCl and 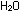 reactions; it is
reactions; it is
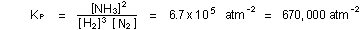
Note how the exponents on each concentration term are related to
the number of molecules in the chemical reaction, and how all of
the exponents determine the overall units of 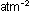 for
for 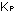 . If concentrations
were given in moles per liter instead of atmospheres, the numerical
value of the equilibrium constant K would be different. . If concentrations
were given in moles per liter instead of atmospheres, the numerical
value of the equilibrium constant K would be different.
The rates of forward and reverse reactions become equal when appreciable
amounts of both reactants and products are still present. In our
crabapple analogy, the two opponents are more evenly matched in
the ammonia reaction, and there is no overwhelming pileup at equilibrium
on either side of the fence. The ammonia reaction is not "complete"
in the way that the other two reactions are.
Photo: The Incitec ammonia synthesis plant, Brisbane, Queensland.
|

|
