|
We now must be careful,
for the numerical value of 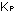 given was for these units only:
given was for these units only:
 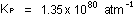
If we used this numerical value but expressed concentrations in
moles per liter, the results of the calculation would be meaningless.
We could just as well have written the  combustion reaction as
combustion reaction as
 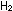 (g) +
(g) + 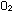 (g) (g)
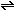 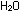 (g)
(g)
 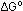 = -54.6 kcal per mole of
= -54.6 kcal per mole of 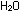
in which case the equilibrium-constant expression would be
   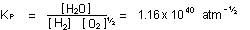
|
|
If we halve the coefficients
of a chemical reaction, we must halve the corresponding free energy
change of the reaction, and take the square root of the equilibrium-constant
expression (which is the same as halving the exponents).
We also must take the square root of the value of the equilibrium
constant,  .
If we double the coefficients of a reaction, we must double the
free energy change and square the equilibrium expression and .
If we double the coefficients of a reaction, we must double the
free energy change and square the equilibrium expression and  . .
Whenever you use either equilibrium constants or free energies,
you must be careful to be sure that you know the chemical reaction
and the concentration units to which the numerical values of  apply.
apply.
|