|
In the water and ammonia
syntheses just discussed, the numbers of molecules of reactants
and products in the equation were different, so  was not a unitless quantity. The conversion of units from atmospheres
to moles per liter is a simple one involving the ideal gas law,
PV = nRT, which we discussed in Chapter 2. In this expression
Pis the pressure in atmospheres, V is the volume in liters, n
is the number of moles, T is the absolute temperature, and R is
the gas constant expressed in units of liter atm per deg per mole:
was not a unitless quantity. The conversion of units from atmospheres
to moles per liter is a simple one involving the ideal gas law,
PV = nRT, which we discussed in Chapter 2. In this expression
Pis the pressure in atmospheres, V is the volume in liters, n
is the number of moles, T is the absolute temperature, and R is
the gas constant expressed in units of liter atm per deg per mole:
 R = 0.08205
liter atm deg R = 0.08205
liter atm deg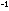 mole
mole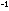
For the jth component in a mixture of gases,
 p p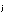 V = n
V = n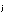 RT
RT
 p p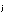 = (n
= (n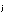 / V) RT =
/ V) RT = 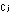 RT RT
in which p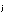 is the partial pressure and
is the partial pressure and 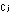 is the concentration of the jth gas in moles per liter.
is the concentration of the jth gas in moles per liter.
Equilibrium constants with concentrations expressed as partial pressures
in atmospheres are designated by 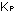 ,
and constants in units of moles per liter are designated by ,
and constants in units of moles per liter are designated by 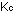 .
For the water formation reaction that we have just seen, these constants
can be written as .
For the water formation reaction that we have just seen, these constants
can be written as
|
|
 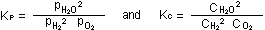
To obtain 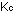 ,
from ,
from 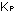 , one need
only substitute p , one need
only substitute p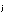 =
= 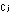 RT for each
chemical substance, reactant and product: RT for each
chemical substance, reactant and product:
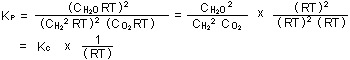
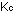 = = 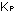 RT = 1.35 x 10
RT = 1.35 x 10 atm
atm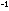 (mole / liter)
(mole / liter)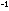
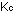 = 1.35 x 10 = 1.35 x 10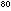 x 0.08205 x 298 liter
x 0.08205 x 298 liter 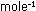
 = 3.31
x 10 = 3.31
x 10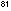 liter
liter 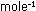
Only if the number of moles of reactants and products is the same,
will 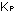 and Kc
be the same unitless number. In general, if the balanced chemical
equation shows an increase of and Kc
be the same unitless number. In general, if the balanced chemical
equation shows an increase of 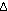 n
in the number of moles of gas in the products as compared with reactants,
then n
in the number of moles of gas in the products as compared with reactants,
then
 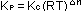
|