Sodium Through Argon
|
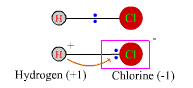
Hydrogen is less electronegative than chlorine, so in HCl the electron pair of the bond is assigned entirely to chlorine to make the pseudo-ionic molecule H+Cl-.
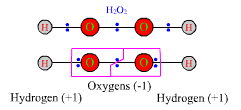
Hydrogen in HCl has an ON of +1, and chlorine has an ON of -1. This pseudo-ionic state becomes real when HCl is dissolved in water and the molecule dissociates. The oxidation numbers, however, are equally valid even for gaseous HCl, which consists of un-ionized H-Cl molecules. If two atoms connected by a bond have identical electronegativities, as in the O-O bond in hydrogen peroxide, H-O-O-H, or in a diatomic gas, F-F, then in calculating oxidation numbers the electron pair is divided between them. Each oxygen in hydrogen peroxide, H2O2, is assigned both |
electrons from its H-O bond and one of the two electrons from the O-O bond. The pseudo-ions corresponding to hydrogen peroxide are H+1 O-1 O-1 H+1. Hence H has an ON of +1, oxygen has an ON of -1, and the total ON for all the atoms in the molecule is zero. Giving one of the bonding electrons to each atom in hydrogen gas, H-H, would produce two neutral atoms, H0 H0, so the ON of each atom is zero. This is true for other diatomic gases such as Cl2, N2, and O2, and in fact is true for any pure element. For a pure element, in which every atom has the same attraction for electrons, each atom has an ON of zero.
|