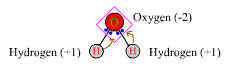Sodium Through Argon
|
A substance such as O, which itself is easily reduced, is a good oxidizing agent for other materials; conversely, a substance that is easily oxidized will be a good reducing agent. How much oxidation can a given amount of a reducible substance accomplish? How many molecules of methane can one molecule of oxygen gas oxidize? For a simple example like this one, the answer can be found merely by balancing the equation, or making sure that there are the same numbers of C, H, and O atoms before and after a reaction. But for more complicated reactions it is helpful to use a concept called the oxidation number. For a single-atom ion, the oxidation number is simply the charge on the positive or negative ion. Thus Na+ has an oxidation number (ON) of +1, and Cl- has an ON of -1. An atom in a metal, such as magnesium, has a zero ON. In calculating oxidation numbers, each mobile electron in the metal is "given back" to its original ion, since all ions in the metal have the same attraction for electrons. |
When magnesium is oxidized to Mg2+, it acquires an ON of +2. Oxidation numbers are merely a means of keeping track of oxidation-reduction processes. Oxidation numbers of atoms are only slightly more complicated to calculate when the atoms are covalently bonded in molecules. In this case, one pretends that the covalent compounds are totally ionic, with each electron pair in a bond being given completely to the more electronegative of the two atoms. Thus in the water molecule, oxygen is more electronegative (EN =3.5) than hydrogen (EN=2.1), and in figuring oxidation numbers, the covalent molecule H-O-H is thought of as H+ O2- H+. The oxidation number of each atom is the net charge on each of the pseudo-ions. Oxygen in water has an ON of -2, and each of the hydrogens has an ON of +1.The sum of the oxidation numbers of all the atoms in a neutral molecule is zero.
|