Sodium Through Argon
|
The bonding electrons in CO2 are pulled closer to O because it is more electronegative than C. Although carbon does not give up its electrons, it does relinquish them to an extent when they shift toward the more electronegative oxygen atoms. If an atom abandons an environment in which its electron pairs are shared equally with a neighbour, and forms a new bond with a more electronegative atom in which the electron pair is more tightly bound to the new neighbour, then we consider that the original atom has been oxidized, even though no actual loss of electrons occurs. In the combustion of methane, C and H atoms combine with oxygen
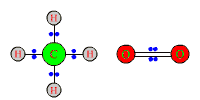
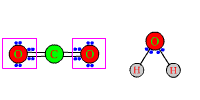
to produce CO2 and H2O: CH4 + 2O2 ------> CO2 + 2H2O The electron pairs in the four bonds of methane initially are shared equally between the almost equally electronegative C and H. Electrons also are shared equally in the O2 molecules, of course, because the two atoms are identical. |
After reaction, carbon and hydrogen each are bonded to the more electronegative atoms of oxygen. The electron pairs in the single and double bonds of H-O-H and O-C-O all will be shifted away from H or C, and closer to O.
Although there has been only a tendency toward removal of electrons instead of outright removal, C and H still have been oxidized. Each O atom has had electrons shifted toward it, and therefore has been reduced. |