Sodium Through Argon
|
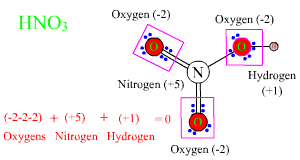
An element can have several different oxidation numbers, or oxidation states, in different compounds. Nitrogen has an ON of zero in N2 gas, and -3 in ammonia, NH3. What is its ON in the nitrate ion? Oxygen is more electronegative than nitrogen, and if all of the bonding electrons are assigned to oxygen, each oxygen atom has a pseudo-ionic charge of -2, and the central nitrogen, a pseudo-ionic charge of +5. This is the charge that each atom would have if nitric acid were a purely ionic compound instead of a covalently bonded molecule. The delocalization of electrons makes no difference. |
If you start from any of the bond models of nitric acid presented in Chapter 4, and assign all of the bonding electrons to the more electronegative of the two atoms bonded, the result will be the same. Oxygen has an ON of -2 in the nitrate ion, and nitrogen has an ON of +5. The total overall ON is the sum of -2, -2, -2, +5 = -1, the same as the charge on the nitrate ion. If we add a proton with an ON of +1, a neutral HNO3 molecule is the result, with an overall ON of zero. Three shortcuts can save a lot of work in figuring out oxidation numbers: 1. Whenever oxygen is bonded only to atoms less electronegative than itself (which rules out only O-F and O-O bonds), oxygen has an ON of -2. 2. Except when bonded to metals in metal hydrides, or to itself in H2, hydrogen has an ON of +1. 3. The sum of the ON of all atoms in a neutral molecule is zero, and the sum of ON of all atoms in an ion is equal to the charge on the ion. |