|
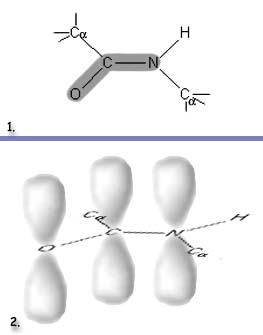
As with other resonance bond structures seen in previous chapters, the actual structure is somewhere in between. The measured bond lengths from protein chains are shown on the bottom drawing of the three. The C-0 distance is close to that expected for a double bond, but the C-N distance is between the single and double bond values, skewed somewhat toward a double bond. The sharing of the nitrogen lone pair with carbon evidently is incomplete, as is the pushing away of the second electron pair from the C=O bond onto the oxygen atom. Oxygen then acquires a slight excess of electrons and a partial negative charge. The electron deficiency created at nitrogen pulls the N-H bonding pair toward N, so the partial positive charge ends up on the hydrogen atom, as shown. From a delocalization viewpoint, the second electron pair of the C=O double bond and the nitrogen lone pair both have been delocalized over the entire O-C-N region (lower right). This delocalization gives the amide group an extra 21 kcal mole-' of stability, which means that one cannot twist the group about the C-N bond without supplying the 21 kcal mole-' necessary to break the partial double bond. There are two important structural consequences of this bonding. The planar amide group may be considered as a rigid structural unit whose only degrees of freedom are swivels about the connections to the alpha carbons, and the 0 and H of the amide plane have slight negative and positive charges that aid in the formation of the hydrogen bonds that hold different chains together. |
|