|
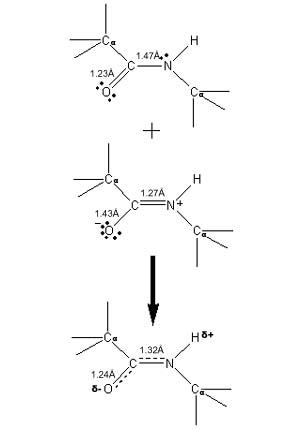
An important feature in the structure of proteins is the fact that all four of the atoms in an amide group, and the two alpha carbons that they connect, must lie in the same plane. This is necessary because the amide bond connecting a C from one amino acid to the N of the next is a partial double bond. The reasons are illustrated at the right. The top drawing shows a conventional bond diagram of an amide group, which has a single C-N bond and a double bond between C and 0. The nitrogen atom has an unused lone electron pair, and the carbonyl oxygen has two more. However, this is only one possible resonance structure for the amide group. The middle drawing shows another equally valid bond arrangement, in which the nitrogen lone electron pair has been donated to the C-N bond to make it a double bond, and one of the C=O double bond electron pairs in turn has been pushed onto the oxygen atom. The nitrogen atom has a net positive charge because of the sharing of its lone electron pair, and the oxygen atom is negative because it now has three lone electron pairs. The bond lengths that would be expected for the C-N and C-0 bonds in each of these two resonance structures are shown. Right: Two possible bonding arrangements in the amide plane in proteins, with the double bond from C to O (top) or from C to N (center). The actual bonding is intermediate (bottom), as the measured bond lengths suggest. |
|