|
|
|
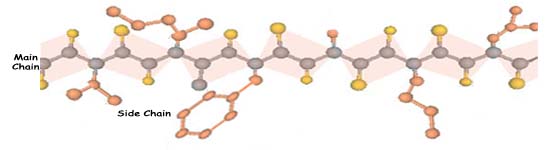
The amide plane as drawn earlier has the two alpha carbons in the trans conformation, at opposite corners of the rectangle. The cis form, with the alpha carbons on the same side of the rectangle, is almost never found in proteins, probably because it introduces a sharp bend in the chain and brings side groups close enough to clash. Above: The amide link (-CO-NH-) is the repeating unit of the main chain in proteins; the side chains vary. In the extended chain above, side chains project alternately to one side and the other. The twenty different amino acid side chains that are coded by DNA are shown on these two pages, grouped according to chemical behavior. The polypeptide main chain with side groupings branching from it appears as a frieze across the top of the opposite page. It is not so important that you remember all of these different side chains as it is that you appreciate the varied chemical properties that they can show. The groups on the opposite page are more or less polar, and tend to be found on the outside of proteins, in contact with water. Aspartic and glutamic acids have carboxylic acid groups (-COOH) on their side chains. These are ionized at pH 7, so aspartic and glutamic acids are means of introducing negative charges onto the surface of a protein molecule. As shown at the bottom of the opposite page, lysine and arginine side chains are bases, which pick up a proton and hence carry a positive charge at neutral pH. The other side chains on the opposite page generally are polar but uncharged. They prefer an aqueous environment for the same reason that methanol molecules do. They help to determine the way a protein chain will fold by tending to keep their parts of the chain on the outside of the molecule. |