|
When several atoms are covalently bonded into an ion such as the
ion goes through many chemical reactions as a unit, behaving like
a single-atom ion of the same charge. Carbonate ions can form salts
with  or other positive ions just as fluoride ions can.
or other positive ions just as fluoride ions can.
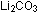 is lithium carbonate and, like
is lithium carbonate and, like 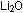 ,
the crystal structure of this salt has two +1 ions ( ,
the crystal structure of this salt has two +1 ions ( )
for every -2 ion )
for every -2 ion 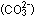 . .
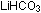 ,
with one positive ,
with one positive  and one positive H+ ion, is known as lithium hydrogen carbonate
(or in an older nomenclature, lithium bicarbonate since it contains
twice as much carbonate per lithium atom as
and one positive H+ ion, is known as lithium hydrogen carbonate
(or in an older nomenclature, lithium bicarbonate since it contains
twice as much carbonate per lithium atom as 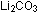 does).
does).
Many negative ions, or anions, are built from several covalently
bonded atoms; the carbonate ion is an example. Positive ions, or
cations, with several atoms are less common, in comparison with
single-atom metal ions such as  or
or 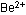 .
.
|
|
One exception that we have seen already is the ammonium ion, 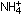 ,
in which four H atoms are covalently bonded around a central nitrogen. ,
in which four H atoms are covalently bonded around a central nitrogen.
Carbonic acid would be intrinsically stronger than boric acid because
of the greater electronegativity of carbon over boron, but delocalization
adds an extra bit of acid strength. There is no delocalization in
the borate ion, 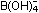 ,
since every electron is either tied up in a specific electron-pair
bond between atoms or occupied as an oxygen lone pair. ,
since every electron is either tied up in a specific electron-pair
bond between atoms or occupied as an oxygen lone pair.
Even with extra help from delocalization, carbonic acid is weak
enough to be drunk in carbonated beverages. Delocalization really
becomes important in increasing acidity in the next molecule to
be discussed, nitric acid.
|