|
Delocalization of electrons helps to make carbonic acid a stronger
acid than boric acid. For benzene, we found that delocalizing the
six ring electrons made the molecule 40 kcal mole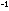 more stable than it would be otherwise.
more stable than it would be otherwise.
The carbonate ion with its two delocalized electrons also is more
stable than it would be if the electrons were confined to the two
oxygens that released protons during dissociation. Dissociation
and association are reversible processes, and both are going on
simultaneously:
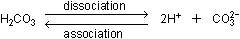
The actual amounts of carbonic acid and carbonate ion that exist
in solution are the result of a balance, or equilibrium, between
the forward and reverse processes.
|
|
Delocalization, by making the carbonate ion more stable, favors
dissociation and discourages association, because the delocalization
is destroyed and the electrons are trapped in O-H bonds when the
protons rejoin the carbonate ion.
This shifts the balance or equilibrium point to the right, thereby
producing more 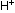 ions, and making carbonic acid a stronger acid than it would be
without delocalization.
ions, and making carbonic acid a stronger acid than it would be
without delocalization.
Another way to look at this behaviour is to recognize that the two
negative charges on a carbonate ion would have the strongest attraction
for protons if they were tied down on specific oxygens on the outside
of the ion.
If the negative charges are spread over the entire ion, the attraction
of the ion for protons is blurred. This discourages association,
and ensures that more protons remain loose than would be the case
if delocalization did not exist.
|