|
Nitrogen has many oxides, with varying degrees of electron sharing.
These oxides range from colorless gases (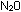 and NO), to a brown gas (
and NO), to a brown gas (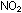 ),
to an explosive white solid ( ),
to an explosive white solid (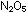 ). ).
If these all were ionic compounds rather than covalent molecules,
and if each oxygen atom took two electrons from a nitrogen atom
to complete its neon shell, then the formulas of the oxides given
above would suggest that nitrogen gave up one electron in 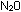 (recall
(recall 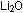 ),
two electrons in NO, four in ),
two electrons in NO, four in 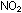 ,
and all five in ,
and all five in 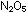 . .
This is not what really happens; electrons in these molecules are
shared rather than given up. But this formal accounting scheme leads
to a useful quantity, the oxidation number of nitrogen, which ranges
from +1 through +5 in these oxides.
The oxidation number is the charge that the nitrogen atom would
have if both electrons in each covalent bond were given to the more
electronegative oxygen atom.
We will return to the important concepts of oxidation and oxidation
numbers in Chapter 6.
|
|
|