|
Because 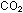 and CO are small molecules with weak van der Waals forces, they
remain gases at ordinary temperatures, like
and CO are small molecules with weak van der Waals forces, they
remain gases at ordinary temperatures, like 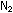 and
and 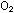 .
Although one can smother in .
Although one can smother in 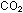 because of the absence of oxygen, carbon dioxide is not intrinsically
poisonous.
because of the absence of oxygen, carbon dioxide is not intrinsically
poisonous.
Carbon monoxide is a different story. Oxygen is picked up at the
lungs and carried to where it is needed in the tissues by hemoglobin,
a protein molecule in the bloodstream. A hemoglobin molecule normally
binds 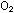 ,
but can be fooled by carbon monoxide, which binds to hemoglobin
even more strongly than oxygen does. ,
but can be fooled by carbon monoxide, which binds to hemoglobin
even more strongly than oxygen does.
Unfortunately for the person who breathes CO, once this happens,
that particular hemoglobin molecule is permanently out of action
and useless thereafter in carrying its proper cargo of 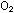 . .
Carbon monoxide thus is poisonous in a sense that carbon dioxide
is not.
|
|
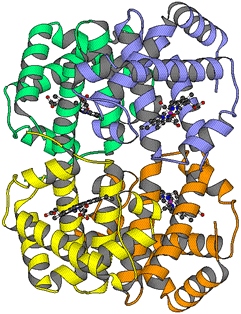 Picture of a
Haemoglobin Molecule
Picture of a
Haemoglobin Molecule |
