|
Apparatus
Magnetic stirrer and follower (optional).
1dm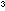 beaker.
beaker.
Chemicals
The quantities given are for one demonstration
75 cm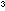 of concentrated sulphuric acid (H
of concentrated sulphuric acid (H SO SO ). ).
9 g of malonic acid (propanedioc acid, CH (CO (CO H) H) ). ).
8g of potassium bromate(V) (KBrO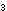 ). ).
1.8g of manganese(II) sulphate (MnSO .H .H O). O).
750 cm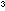 of deionised water.
of deionised water.
Before the Demonstration
Place 750 cm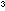 deionised water in a 1 dm
deionised water in a 1 dm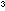 beaker. Slowly, and with stirring, add 75 cm
beaker. Slowly, and with stirring, add 75 cm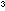 concentrated sulphuric acid carefully. The mixture will heat up
to about 50
concentrated sulphuric acid carefully. The mixture will heat up
to about 50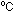 .
Allow it to cool back to room temperature. This will take some time.
Weigh out the malonic acid, potassium bromate and manganese sulphate
in weighing boats. .
Allow it to cool back to room temperature. This will take some time.
Weigh out the malonic acid, potassium bromate and manganese sulphate
in weighing boats.
|
|
The Demonstration
Place the beaker of sulphuric acid on a magnetic stirrer and stir
the solution fast enough for a vortex to form. A stirring rod can
be used, but it is tedious and tends to detract from the demonstration.
Add the malonic acid and potassium bromate.
When these have dissolved, add the manganese sulphate and observe
what happens. A red colour should develop immediately. This will
disappear after about one minute and thereafter the colour will
oscillate from red to colourless with a time period of about 20
seconds for a complete oscillation.
This will continue with a gradually increasing time period for over
ten minutes - long enough for most audiences to lose interest!
|