|
Theory
This reaction is an example of a class of reactions called Belousov-Zhabotinsky
(BZ) reactions. The overall reaction is usually given as:
3CH (CO (CO H) H) (aq)
+ 4BrO (aq)
+ 4BrO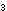  (aq) (aq)
 4Br 4Br (aq)
+ 9CO (aq)
+ 9CO (g) (g)
 + 6H + 6H O(l) O(l)
Oscillation is brought about by two autocatalytic steps. Bromine
is an intermediate in the reaction scheme - the red colour that
is observed. An analogy with predator-prey relationships might be
one way to give students some idea of what is going on. For example
a population of rabbits (analogous to the bromine) will increase
rapidly (exponentially) if there is plenty of food (reactants).
However, the plentiful supply of rabbits will stimulate a rapid
increase in the fox population (another intermediate that reacts
with bromine) which will then deplete the rabbits. Lacking rabbits,
the foxes will die, bringing us back to square one, ready for a
rapid increase in rabbits and so on.
|
|
Extensions
The reaction can be investigated using a colorimeter with a
chart recorder or interfaced to a computer.
Further details
The reaction will not work if tap water (Coventry) is used instead
of deionised water. Chloride ions, via the addition of a pinch of
potassium chloride or dilute hydrochloric acid will immediately
stop the oscillations. Clean apparatus is therefore essential.
Safety
Wear eye protection.
The reaction mixture can be washed down the sink with plenty of
water after the demonstration.
|