|
Apparatus
1dm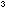 measuring cylinders - as many as the number of indicators to be
used.
measuring cylinders - as many as the number of indicators to be
used.
Expanded polystyrene cool box to store the dry ice. The type of
box in which Winchester bottles are often supplied is ideal.
Chemicals
The quantities given are for one demonstration
A few cm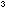 of universal indicator and/or other indicators as desired.
of universal indicator and/or other indicators as desired.
A few cm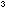 of dilute ammonia solution and/or dilute sodium hydroxide
solution
of dilute ammonia solution and/or dilute sodium hydroxide
solution
About 100g or dry ice (solid carbon dioxide). This should be bought,
since dry ice made from a carbon dioxide cylinder attachment will
float and is less effective at saturating the solutions. Dry ice
can be obtained from universities, higher education institutions,
hospitals and industry. It can be stored and transported in expanded
polystyrene boxes.
|
|
The Demonstration
Fill a 1 dm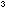 measuring cylinder to the 1 dm
measuring cylinder to the 1 dm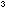 mark with water and add enough universal indicator to give an easily
visible colour.
mark with water and add enough universal indicator to give an easily
visible colour.
Add a few drops of either ammonia solution or sodium hydroxide solution,
to make the water alkaline, and stir. Add a few lumps of dry ice.
These will sink to the bottom and bubble as CO is given off. A spectacular fog will be produced at the top of the
cylinder and, after several minutes, the indicator will change colour
from blue to orange.
is given off. A spectacular fog will be produced at the top of the
cylinder and, after several minutes, the indicator will change colour
from blue to orange.
The colour change will be more gradual if ammonia is used as the
alkali, as the reaction that occurs is a weak acid - weak base reaction.
The final pH reached is about 4.5 and therefore, if colour changes
that take place at pH's lower than this are to be shown, it will
be necessary to add a little concentrated hydrochloric acid at the
end.
|