|
Theory
The relevant neutralisation equations are:
with ammonia
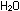 (l) + (l) + 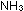 (aq)
+ (aq)
+ 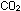 (g) (g)
 NH NH 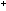 (aq)
+ HCO (aq)
+ HCO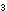  (aq) (aq)
with sodium hydroxide
OH (aq)
+ (aq)
+ 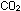 (g) (g)
 HCO HCO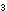  (aq)
(aq)
|
|
Extensions
Other indicators can be used such as phenolphthalein (pink to
colourless), thymolphthalein (blue to colourless), thymol blue (blue
to yellow), phenol red (red to yellow) and bromothymol blue (blue
to yellow).
Safety
Wear eye protection and use gloves to handle the dry ice as it can
cause frostbite burns.
|