|
Apparatus
Trigger pump operated spray bottles such as those used for spraying
house plants. Products such as window cleaner are sold in these
bottles and empty ones can be cleaned with water and re-used for
this experiment. Ideally, one bottle is needed for each metal, although
it is possible to wash one out between solutions.
Chemicals
The quantities given are for one demonstration.
About 10 cm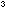 of ethanol for each metal.
of ethanol for each metal.
Less than 1g of a salt of each metal. Chlorides are best, but other
salts are worth trying if chlorides are not available. Examples
of compounds that work well are: sodium chloride, potassium chloride,
lithium iodide and copper sulphate.
|
|
Before The Demonstration
Make a saturated solution of each salt in about 10cm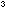 of ethanol. Only a few mg of each is required. Place each solution
in a spray bottle and label it.
of ethanol. Only a few mg of each is required. Place each solution
in a spray bottle and label it.
The Demonstration
Adjust the nozzles of the spray bottles to give a fine mist
and spray the solutions into a roaring bunsen flame. Take care to
spray away from yourself and the audience. The colour of the resulting
jet depends on the metal used. The solutions can be retained for
future use and can be stored in the plastic bottles for several
weeks, at least, without apparent deterioration of the bottles.
Students can observe line spectra through hand held spectroscopes.
|