|
Apparatus
One 1dm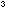 conical flask with stopper.
conical flask with stopper.
Chemicals
The quantities given are for one demonstration.
8g of potassium hydroxide or 6g of sodium hydroxide.
10g of glucose (dextrose).
0.05g of methylene blue.
50cm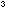 of ethanol.
of ethanol.
|
|
Before The Demonstration
Make a solution of 0.05g of methylene blue in 50cm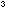 ethanol (0.1%).
ethanol (0.1%).
Weigh 8g of potassium hydroxide or 6g of sodium hydroxide into a
1dm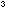 conical flask. Add 300 cm
conical flask. Add 300 cm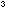 of water and 10g of glucose and swirl until the solids are dissolved.
Add 5cm
of water and 10g of glucose and swirl until the solids are dissolved.
Add 5cm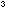 of the methylene blue solution. None of the quantities is critical.
The resulting blue solution will turn colourless after about one
minute. Stopper the flask.
of the methylene blue solution. None of the quantities is critical.
The resulting blue solution will turn colourless after about one
minute. Stopper the flask.
The Demonstration
Shake the flask vigorously so that air dissolves in the solution.
The colour will change to blue. This will fade back to colourless
over about 30 seconds. The more shaking, the longer the blue colour
will take to fade. The process can be repeated for over 20 cycles.
After some hours, the solution will turn yellow and the colour changes
will fail to occur.
|