|
The
corresponding equation for acidic solutions can be obtained by adding
enough hydrogen ions to each side to eliminate the hydroxide ions:
 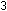 
 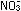
 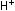
 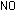
 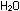
 
 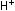 
   
   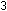 
 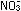
 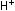
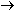 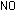
 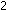 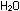
    
Example.
Potassium permanganate, 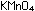 ,
is a common inorganic oxidizing agent, which becomes reduced to
manganous ion, Mn ,
is a common inorganic oxidizing agent, which becomes reduced to
manganous ion, Mn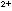 ,
in acidic solution. Write a balanced equation for the reaction in
which permanganate oxidizes ferrous iron, Fe2+,
to ferric ion, Fe3+. ,
in acidic solution. Write a balanced equation for the reaction in
which permanganate oxidizes ferrous iron, Fe2+,
to ferric ion, Fe3+.
Solution.
 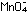
 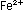
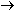 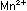
 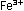
In the permanganate ion manganese has an oxidation number of +7
which decreases to +2 in the manganous ion, a change of -5. Iron
increases in oxidation number by one:
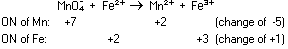
|
The
tetrahedral permanganate ion
Formula MnO4-
|