|
It is obvious that five iron
atoms are required for every manganese atom, in order that
the changes in oxidation numbers cancel:
 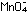
 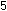 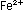
 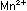
 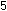 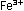
    
Eight hydrogen ions are needed to use up the four oxygen atoms
on the left, leading to four water molecules on the right:
|
|

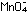
 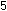 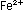
 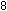 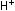
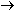 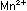
 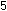 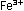
 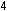 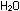

In choosing a 1-to-5 ratio of Mn-to-Fe, we ensured that oxidation
number was conserved. By adding the hydrogen ions we obtained
an equation that balanced the number of each atom. As a final
check, the net charge on each side of the equation can be
tested, and found to be the same: +17. This oxidation-reduction
equation now is balanced with respect to electrons,
atoms, and charge.
|