|
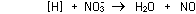
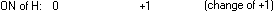

For electrons to balance properly, three moles of H must be oxidised
for every mole of NO3- reduced:
3 (+1) + +
 (-3) (-3) =
= 0 0
and the reaction can be written
 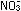
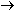 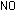
 
    
    
    
In any reaction in aqueous solution, one is at liberty to assume
as many H2O,
H+, or OH-
as are required to balance the reaction, using H2O
and H+ under acidic
conditions, or 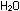 and OH- if the solution
is basic. The redox (oxidation - reduction) part of the balancing
has been done, and what remains is only an accounting for O and
H atoms. One possible answer is
and OH- if the solution
is basic. The redox (oxidation - reduction) part of the balancing
has been done, and what remains is only an accounting for O and
H atoms. One possible answer is
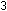 
 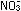
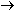 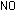
 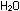
 
    
    
This has accounted for the three hydrogen atoms and the negative
charge. The equation is correct for basic solutions where OH-
ions are present.
|
The
planar, delocallised nitrate ion
Formula
NO3-
|