|
How can we write a properly balanced equation for the oxidation
of foods with NO as the waste product?
 
 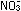
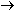 
 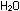  
It might be tempting to balance by inspection along the following
lines, making the number of each kind of atom the same on
left and right:
|
|
 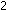 
 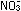 
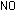
 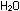
 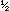 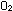        
But this leaves an electron on the left side unaccounted for
on the right side. The approach that is most nearly foolproof
and at the same time shows you what is happening is the oxidation-number
method. In nitrates, N has an oxidation number of +5 ( verify
this for yourself ). In NO the oxidation number of N is +2,
a decrease of three. Hydrogen, as before, goes from 0 to +1,
an increase of one:
|