|
These
oxidation numbers (ON) of H and O in H2O arise because O is more
electronegative than H, so both the electrons in each O H
bond are assigned to O. If you are unsure of this process, look
back at Chapter 6. This is such a simple chemical reaction that
it can be balanced by inspection - by making sure that the same
number of atoms of H and O are on each side of the equation. The
balanced equation is H
bond are assigned to O. If you are unsure of this process, look
back at Chapter 6. This is such a simple chemical reaction that
it can be balanced by inspection - by making sure that the same
number of atoms of H and O are on each side of the equation. The
balanced equation is
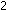 
 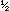 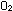
 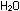
 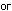
 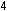 
 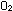
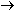 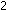 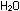   
We also could have balanced the equation by seeing to it that the
net change in oxidation number of all substances was zero. If the
oxidation number of one oxygen atom decreases by two, then two hydrogen
atoms each must increase by one. In physical terms, if one oxygen
atom pulls two electrons toward itself, then two hydrogen atoms
are required to donate one electron each. In terms of changes in
oxidation number,
   2H 2H
 O O
Changes in ON:   2(+1) 2(+1)
  + +
 (-2) (-2) =
= 0 0
This was a trivial example, but the following example is not quite
so trivial. If oxygen is in short supply, some bacteria can respire
using nitrates as sources of oxidizing power instead of O2.
Rather than reducing oxygen to water, these bacteria reduce nitrates
to NO2 , NO, or N2
.
|
|