|
11.
Since the formation of water from its elements is highly spontaneous,
how is it possible to collect the product gases as described in
Question 10? Is the mixture of  and
and 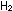 gases at equilibrium? How can they be made to come to equilibrium
again, and what would be the resulting substance?
gases at equilibrium? How can they be made to come to equilibrium
again, and what would be the resulting substance?
12. If the gases obtained as described in Question 10 are
collected in a tank that then is sealed, and if the tank has a total
interior pressure of 0.5 atm, what is the partial pressure of each
gas?
13. Which reaction is more spontaneous, starting from standard
conditions, the synthesis of HCl, 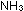 ,
or ,
or 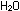 ?
In which reaction will more of the starting materials remain unreacted
when equilibrium has been reached? ?
In which reaction will more of the starting materials remain unreacted
when equilibrium has been reached?
14. What is the difference between 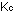 , ,
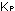 ,
and ,
and 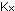 ?
What is the relationship between ?
What is the relationship between 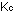 and
and 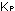 for the ammonia synthesis reaction when written as
for the ammonia synthesis reaction when written as 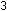 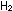 +
+ 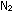
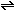
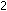 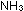 ?
?
15. What is the general expression relating 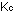 and
and 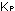 for gases?
for gases?
|
|
16.
Dalton's law of partial pressures tells us that partial pressure
is related to mole fraction in a gas mixture by the expression
p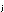 = P = P 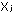 ,
in which P is the total pressure. What is the general expression
relating ,
in which P is the total pressure. What is the general expression
relating 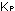 and and
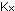 for gases? for gases?
17. What is Le Chatelier's principle? What would it indicate
about the effect on an equilibrium constant of raising the temperature
at which the reaction took place?
18. How can Le Chatelier's principle predict the effect on
equilibrium concentrations of a change in overall pressure?
19. Does a change in pressure bring about a change in the
numerical value of the equilibrium constant, 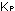 ?
Does a change in temperature lead to a change in numerical value
of ?
Does a change in temperature lead to a change in numerical value
of 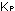 ? ?
20. If a reaction has a large negative standard free energy
change, is the reaction spontaneous if started from standard conditions?
What is meant by standard conditions? Will the equilibrium constant
for this reaction be large, or small, and how is it related to the
standard free energy change?
|