|
1.
What is the difference between the spontaneity and the rate of a
chemical reaction? Give an example of a spontaneous but slow chemical
reaction.
2. How can a spontaneous but slow reaction be accelerated?
3. What is the form of the equilibrium-constant expression
for the reaction 2NO (g) +  (g)
(g) 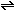
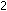 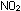 (g) ? Justify the exponents that you use on the concentration terms.
(g) ? Justify the exponents that you use on the concentration terms.
4. The reaction of Question 3 just as easily could be written
NO(g) + 1/2 (g)
(g) 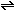
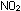 (g).
What is the equilibrium-constant expression for the reaction written
in this way? How are the numerical values of the (g).
What is the equilibrium-constant expression for the reaction written
in this way? How are the numerical values of the 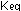 in this question and Question 3 related?
in this question and Question 3 related?
5. Write the equilibrium-constant expression for the reaction
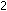 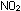 (g) (g)
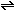 2NO (g) +
2NO (g) +  (g).
How does the numerical value for this equilibrium constant and that
in Question 3 compare? (g).
How does the numerical value for this equilibrium constant and that
in Question 3 compare?
6. What are the units for the equilibrium constants in Questions
3, 4, and 5?
|
|
7.
Why is it reasonable that the rate constant for the first-order
process in the crabapple war should have units of square feet per
second, and in what sense is this an "agility constant"? Why should
it also be reasonable for the first-order rate constant in molecular
processes to have units of liters per second?
8. What principle about the meaning of equilibrium is used
to relate the equilibrium constant to the forward and reverse rate
constants?
9. Does a state of equilibrium mean that all activity on
the molecular level has stopped? If not, then what does equilibrium
mean?
10. What is the definition of mole fraction for a mixture
of gases? If pure water is decomposed by electrolysis, and the resulting
gases are collected as a mixture, what is the mole fraction of each
component?
|