|
5. A
1-liter tank contains 0.095 mole of ammonia, 1.13 moles of nirogen
gas, and 1.5 moles of hydrogen gas at equilibrium.
(a) Calculate 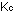 for the reaction
for the reaction
 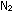 +
+ 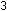 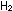
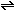
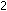
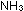 . .
(b) Calculate 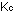 for the reaction
for the reaction
 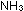
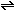 1/2
1/2 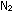 + 3/2
+ 3/2 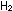 . .
(c) How are the 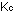 's
calculated for parts (a) and (b) related? 's
calculated for parts (a) and (b) related?
(d) Calculate 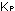 for the reaction as written in part (a).
for the reaction as written in part (a).
6. 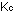 for the reaction
for the reaction 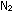 +
+ 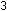 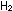
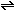
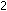
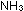 is 0.00237 at 1000K. If a 10-liter steel tank at this temperature
contains 20 moles of
is 0.00237 at 1000K. If a 10-liter steel tank at this temperature
contains 20 moles of 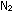 and
30 moles of and
30 moles of 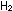 ,
how many liters of ammonia will be present? ,
how many liters of ammonia will be present?
7. For the reaction of Problem 6, in a 10-liter tank at 1000K
what will be the equilibrium concentration of hydrogen gas, if 6.8
moles of nitrogen and 10.5 moles of ammonia are present? How many
moles of hydrogen gas will be present?
|
|
8. The equilibrium
constant for the reaction
PCl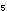 (g) (g) 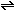 PCl
PCl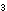 (g) + (g) + 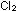 (g)
is (g)
is 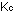 = 0.0224
at 500K. = 0.0224
at 500K.
(a) What are the proper units for this 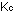 ? ?
(b) What is the numerical value for 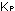 ,
and what are its units?(c)
How many moles of ,
and what are its units?(c)
How many moles of 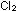 will be present at equilibrium in a 100-liter tank at 500K, if there
are 4.3 moles of PCl
will be present at equilibrium in a 100-liter tank at 500K, if there
are 4.3 moles of PCl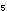 and 132 moles of PCl
and 132 moles of PCl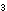 ? ?
(d) If the pressure on the contents of the tanks is increased, will
more, or less, 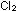 be present at equilibrium?
be present at equilibrium?
(e) If the temperature of the tank is raised, will more, or less,
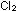 be present? be present?
9. The equilibrium constant for the dissociation of gaseous
iodine molecules at 1000K is 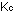 = 3.76 x 10
= 3.76 x 10 for the reaction
for the reaction
I (g) (g) 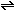 2I (g). An experiment is begun by placing 1.00 mole of pure I
2I (g). An experiment is begun by placing 1.00 mole of pure I in a 2.00-liter box at 1000K. How much atomic iodine will be present
after the contents have come to equilibrium?
in a 2.00-liter box at 1000K. How much atomic iodine will be present
after the contents have come to equilibrium?
|