|
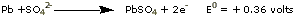
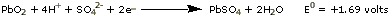
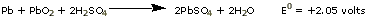
Six-volt and twelve-volt automobile batteries are obtained
by connecting three or six of these cells in series.
When the lead storage battery has run down, most of the
lead and lead oxide is in the form of
lead sulfate, and the battery fluid is
depleted of sulfuric acid.
The fluid then is less dense,
which is the reason that the specific gravity (or density)
of the battery fluid can be used by a service station attendant
as a measure of the state of charge of the battery.
If a direct current is passed through a run-down battery
so electrons flow into the anode (originally Pb) and out the
cathode (originally PbO2), the half-reactions are
reversed.
Lead sulfate is reconverted
to Pb and PbO2, the battery fluid becomes a more
concentrated (and denser) sulfuric acid solution, and
electrochemical energy is stored in the cell, ready for later
use.
|