|
Standard reduction potentials
for various half-reactions under acidic and basic conditions
are given in the linked tables
on the facing page. Remember that these potentials
really are for cells in which the electrons required
on the left side of the given half-reaction are provided
by oxidizing H2 to H+ ions, but
that whenever we use two of these half-reactions to
calculate the potential of a real cell, the hydrogen
contributions cancel. Also remember that a potential
is a "pressure" on a
per electron basis, no matter how many electrons
appear in the half-reaction. The free energy for that
half-reaction coupled in a cell with the hydrogen half-reaction
can be found by multiplying the reduction potential
by -23.06 kcal times the number of electrons involved
in the half-reaction.
Example. What
are the cell voltage and free energy change in a cell
with the reaction
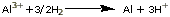
Which way will the reaction go spontaneously?
Solution
Now find out how to Build
cells from Half Rections
|