|
One cannot make a solid electrode out of hydrogen gas, of
course, but the same effect can be achieved by bubbling a
stream of hydrogen gas over an inert conductor such as a platinum
electrode, as shown opposite.
The H2 molecules can dissociate at the surface
of the platinum, give up their electrons to the external circuit
through the metal electrode, and go into solution as H + ions.
Similar electrodes can be made with other gases.
In thinking about standard cell reactions, it is customary
to forget that they are paired with the hydrogen half-reaction,
and to speak of them as if they were isolated reactions of
one half of the cell.
We can talk about half-reactions, and their free energies
and half-cell potentials, as though the following had physical
reality:
|
The potentials as written above are reduction
potentials, positive
if the ion is easier to reduce than
H+ ions, and negative
if harder to reduce than H+.
A large positive reduction potential for a half-reaction is
a sign that the reduced form of the substance is strongly
favored, as with copper in the above examples.
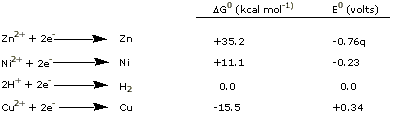
|