|
The half-reactions are
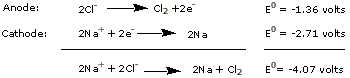
The electrode potentials used here are only approximate because
they are for dilute aqueous solutions,
not for a fused salt. They do indicate that a large
potential must be applied across the electrodes of
the cell before electrolysis
and decomposition of
the salt will begin.
Electrolysis is the only practical
process for obtaining aluminum metal
from its ores, primarily Al203.
Many other metals are either obtained or purified by electrolytic
cells. For example, if a current is passed through
two copper electrodes immersed in a copper sulfate solution,
copper will be oxidized to Cu2+ ions at the anode,
and Cu2+ ions will be reduced and will plate as
metallic copper on the cathode. If an ingot of impure copper
is used as the anode,
then pure copper will build up on the cathode,
and impurities will settle to the bottom of the electrolysis
tank. In a similar way, electrolysis can be used to plate
metal on any object that can be made to conduct electricity
and hence can be used as the cathode in an electrolysis cell.
|
|